Abstract
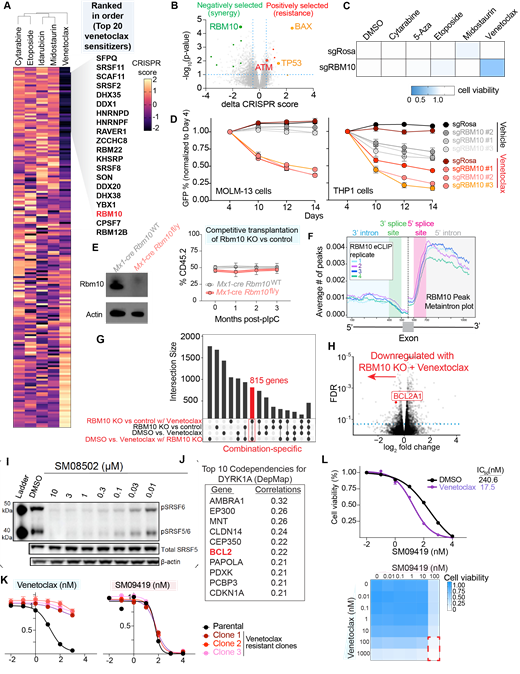
Resistance to therapy is one of the most significant challenges in the treatment of acute myeloid leukemia (AML). While great efforts have uncovered genetic mechanisms of resistance to certain AML-directed therapies, to date, treatment resistance in AML has only partially explained by acquired genetic alterations. Here, we performed genome-wide CRISPR/Cas9 screens to identify drug-gene interactions that modulate therapeutic response to treatments commonly used in AML. Interestingly, our findings uncovered several genes that regulate pre-mRNA splicing whose loss strongly synergized with venetoclax, a BH3 mimetic that blocks the antiapoptotic protein BCL-2. To further delineate the role of RNA processing in response to AML treatments, we performed secondary CRISPR screens with a domain-focused gRNA library targeting 490 RNA processing factors in the presence of various AML drugs. Overall, these genetic screens identified a number of RNA splicing factors whose loss-of-function sensitized AML cells to BCL2 inhibition (Fig. A).
Among the top gene candidates whose loss promoted venetoclax efficacy was the splicing factor RBM10 (Fig.B). Strikingly, loss of RBM10 exclusively synergized with venetoclax-based treatments across AML therapeutics, including in TP53 mutant lines (Fig.C-D). Moreover, RBM10 loss restored venetoclax sensitivity to AML cell line variants with acquired venetoclax resistance. Interestingly, while many RNA splicing factors are pan-essential, generation of an Rbm10 conditional knockout mouse revealed that Rbm10 is completely dispensable for steady-state normal hematopoiesis (Fig.E). Since RBM10 has not been studied previously in hematopoiesis, we mapped the impact of RBM10 on mRNA expression and splicing using RNA-seq and direct RNA binding partners genome-wide by eCLIP-Seq (Fig. F). RBM10 loss was strongly associated with downregulation of BCL2A1, an anti-apoptotic factor whose expression is correlated with venetoclax resistance in AML (Fig.G-H). This was dependent on RBM10's ability to bind RNA and expression of BCL2A1 cDNA fully rescued the growth-inhibitory effect of RBM10 KO-venetoclax treated AML cells. Overall, the above data support RBM10 as a synthetic lethal vulnerability in venetoclax therapy.
Beyond RBM10, our genetic screens also identified several splicing factors belonging to the family of serine and arginine-rich (SR) proteins whose loss synergized with venetoclax treatment (Fig. I). SR proteins are essential for pre-mRNA splicing and are substrates for phosphorylation by conserved family of kinases, such as Cdc2-like kinases (CLKs) and (dual-specificity tyrosine-regulated kinases) DYRKs. We therefore utilized a series of selective pan-CLK/DYRK1A inhibitors, including SM09419 and SM08502, that potently suppress SR protein phosphorylation. Interestingly, BCL2 is one of the top genetic dependencies upon DYRK1A genetic suppression in prior work from the DepMap (Fig. J). Pharmacologic inhibition of CLK/DYRK1A exhibited high in vitro efficacy at nanomolar range across a diverse range of AML subtypes including cell lines with acquired venetoclax resistance (Fig.K). Consistent with this, combined SM09419 and venetoclax displayed synergistic anti-leukemic effects and venetoclax-sensitive AML cell lines (Fig.L). Taken together, these data support the notion of targeting CLK/DYRK1A in the context of BCL2 inhibition.
In this study, we systematically defined gene interactions that mediate the response to a wide range of AML drugs. Recent studies have begun to show that dysfunctional RNA processing promotes AML development. However, the role of RNA processing in modulating drug responsiveness in AML is not well understood. Here, we have uncovered that synthetic lethal targeting of splicing factors, such as RBM10, increases sensitivity of AML cells to BCL2 inhibition. Therapeutically, pharmacologic inhibition of SR protein function via inhibiting CLK/DYRK1A-mediated phosphorylation of splicing factors is an effective strategy used in combination with venetoclax or to overcome venetoclax resistance. Overall, our findings underscore the central importance of RNA splicing in drug response and provides a therapeutic rationale for modulating RNA splicing to enhance current AML therapies.
McMillan: Prizer: Ended employment in the past 24 months. Bossard: Biosplice Therapeutics: Current Employment. Aifantis: AstraZeneca: Research Funding; Foresite (FL2020-010) LLC: Consultancy. Abdel-Wahab: H3B Biomedicine: Consultancy, Research Funding; Foundation Medicine Inc: Consultancy; Merck: Consultancy; Prelude Therapeutics: Consultancy; LOXO Oncology: Consultancy, Research Funding; Lilly: Consultancy; AIChemy: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees; Envisagenics Inc.: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal